SedimentPFCs (µg/kg dry weight)
|
MusselsPFCs (µg/kg wet weight)
|
|
|---|---|---|
|
|
North |
|
| South |
What are they?
Perfluorinated compounds (PFCs) are synthetic compounds used to make products more resistant to stains, grease, and water, and also to help reduce friction. PFCs are found in a wide range of products, including electronic parts, firefighting foam, photo imaging, hydraulic fluids, and textiles (e.g., waterproof outdoor gear).1,2 Key PFCs include perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA). PFOS is the main ingredient in many stain repellants. The salts of PFOA are used to produce fluoropolymers, the large molecules used to make coatings that are water and stain resistant, used in textiles, carpets, and cookware.
How do they get into the ocean?
PFCs are released to the environment during manufacture, and during use and disposal of PFC-containing products (e.g., via landfills and wastewater). Some PFCs are volatile and can be transported in the atmosphere far from the location of release, while others, such as PFOS and PFOA, have low volatility and high water solubility, and can travel long distances with oceanic currents.3
PFCs are persistent in sediments and bioaccumulate in fish, birds, and marine mammals. Unlike other contaminants, PFCs appear to accumulate through waterborne exposure, rather than through the diet.3 PFCs are not stored in body fat; however, they do bind to certain proteins and are slow to be eliminated from organisms.3,4
PFOS is the most common PFC found in environmental samples and is particularly prevalent in the tissues of aquatic organisms. In wildlife, PFOS accumulates primarily in the blood and liver.3
Are they a problem?
Toxicity of PFCs includes possible effects on the immune system, hormones and the liver. Disruption of normal hormone activity and neonatal development have been documented in laboratory animals.4,5 Toxicity of PFOS to aquatic organisms, including plants, invertebrates, and fish has also been documented.3
Additional research is needed to better understand possible adverse effects of PFCs on human health.4
FACT: The firefighting foam used at U.S. military bases and airports was found to be a major source of PFOA and PFOS contamination in local drinking water, affecting the water used by more than 6 million Americans.6
What is being done?
Neither PFOS nor PFOA, their salts, nor their precursors were ever manufactured in Canada 1,2, and all have been added to the Toxic Substances List under the Canadian Environmental Protection Act, 1999. 7 The manufacture, use, sale, and import of PFOA and PFOS is prohibited with specific exemptions where these compounds are present in manufactured items. As of 2002, PFOS is no longer manufactured in the United States, and PFOA production is scheduled for elimination.4 Internationally, PFOS has been added to the Stockholm Convention on Persistent Organic Pollutants, which aims to reduce production and use of PFOS globally.
Environment and Climate Change Canada has developed Federal Environmental Quality Guidelines for PFOS protective of aquatic life and animals consuming aquatic life (Table 1).
Table 1. Federal Environmental Quality Guidelines for PFOS 1 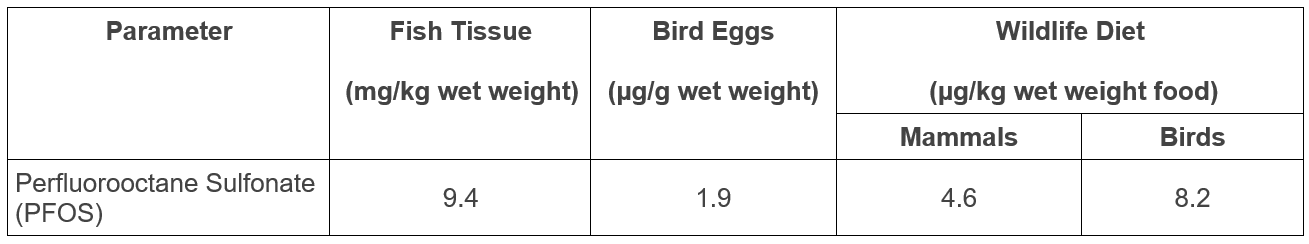
What can we do?
As individuals and organizations, we can:
- Learn more about perfluorinated compounds and other contaminants of concern using the resource links below.
- Recycle and dispose of waste responsibly and according to local guidelines.
- Avoid using products that contain PFCs and other contaminants of concern.8 For example, to decrease exposure to PFCs:
- Avoid stain-repellant treatments for carpets and upholstery in your home and workplace.
- Investigate before you buy. Some outdoor clothing companies offer PFC-free options, or have committed to eliminating PFCs from their waterproof products in the future.
More information?
1 Environment and Climate Change Canada (ECCC). 2017. Canadian Environmental Protection Act, 1999. Draft Federal Environmental Quality Guidelines Perfluorooctane Sulfonate (PFOS). Available at: http://www.ec.gc.ca/ese-ees/default.asp?lang=En&n=38E6993C-1
2 Environment and Climate Change Canada (ECCC) & Health Canada. 2012. Proposed Risk Management Approach for Perfluorooctanoic Acid (PFOA), its salts, and its precursors and long-chain (C9-C20) perfluorocarboxylic acids (PFCAS), their salts and their precursors. Available at: http://www.ec.gc.ca/ese-ees/default.asp?lang=En&n=451C95ED-1
3 Giesy JP, Naile JE, Khim JS, Jones PD, Newsted JL. 2010. Aquatic Toxicology of Perfluorinated Chemicals. In: Whitacre DM (Ed.), Reviews of Environmental Contamination and Toxicology 202: 1-52.
4 National Institute of Environmental Health Sciences (NIH). 2016. Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS). Available at: https://www.niehs.nih.gov/health/materials/perflourinated_chemicals_508.pdf
5 Corsini E, Luebke RW, Germolec DR, DeWitt JC. 2014. Perfluorinated compounds: Emerging POPs with potential immunotoxicity. Toxicology Letters 230: 263-270.
6 Hu XC, Andres DQ, Lindstrom AB, Bruton TA, Schaider LA, Grandjean P, Lohmann R, Carignan CC, Blum A, Balan SA, Higgins CP, Sunderland EM. 2016. Detection of Poly- and Perfluoroalkyl substances (PFASs) in U.S. Drinking water linked to industrial sites, military fire training areas, and wastewater treatment plants. Environmental Science and Technology Letters 3: 344-350.
7 Government of Canada. 2021. Canadian Environmental Protection Act, 1999. (S.C. 1999, c.33). Available at: Canadian Environmental Protection Act, 1999 (justice.gc.ca)
8 Green Science Policy Institute. 2017. Consumers’ Guide to Highly Fluorinated Chemicals. Available at: http://greensciencepolicy.org/highly-fluorinated-chemicals/